Mechanical Circulatory Support Devices Market Forecasted to Exceed $3.45 Billion Globally by 2025: Cardiac Care Expansion in the U.S., Germany, and South Korea
Explore the growing Mechanical Circulatory Support Devices Market as innovative technologies and rising cardiovascular diseases drive substantial industry expansion through 2025.
- Last Updated:
Mechanical Circulatory Support Devices Market in Q1 and Q2 of 2025
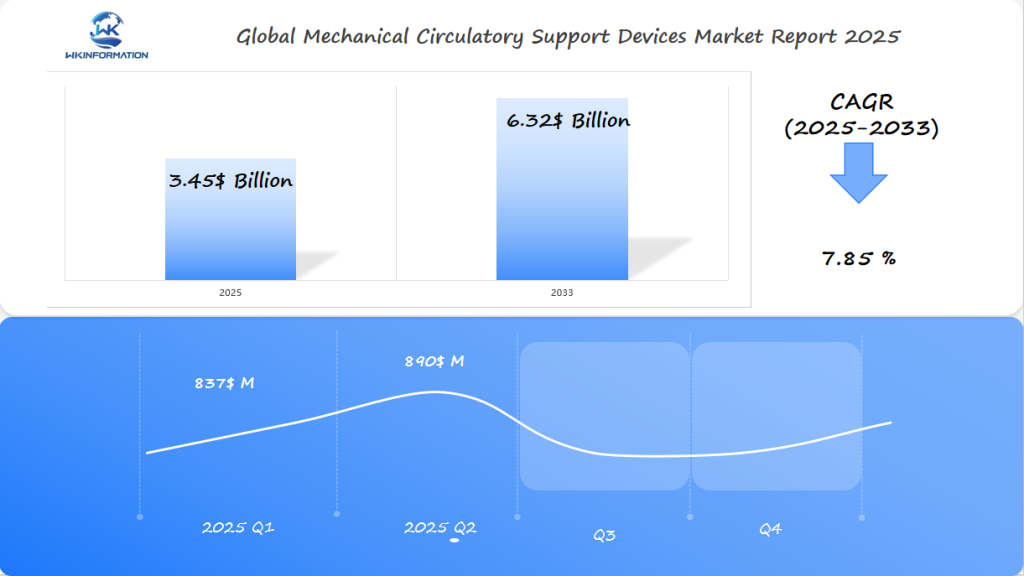
The Mechanical Circulatory Support Devices market is forecast to reach $3.45 billion in 2025, with a robust CAGR of 7.85% from 2025 to 2033. Q1 revenue is anticipated at $837 million, rising to $890 million in Q2. The U.S. dominates due to strong cardiac care infrastructure and Medicare-supported LVAD (left ventricular assist device) programs. Germany continues to expand its implantable device usage across public hospitals, driven by aging demographics and heart failure incidence. In South Korea, investments in minimally invasive cardiac surgery and localized device R&D are boosting adoption. The market is advancing via magnetically levitated pumps, wireless energy transmission, and AI-based patient monitoring platforms.
Key Takeaways
- The market is expected to reach $3.45 billion by 2025.
- Technological innovations are driving device development.
- There is an increasing prevalence of cardiovascular diseases.
- There is a growing demand for minimally invasive procedures.
- Advanced cardiac technologies are leading to enhanced patient outcomes.
Overview of the Upstream and Downstream Industry Chain for Mechanical Circulatory Support Devices Market
The mechanical circulatory support devices market is complex. It involves many players who help make and distribute life-saving heart technologies.
Key Players in the Upstream Supply Chain
Upstream suppliers are key in this chain. They include:
- Advanced material manufacturers
- Precision engineering component producers
- Biomedical research institutions
- Medical-grade semiconductor developers
Research and development partners work with these suppliers. Together, they create new solutions. Their work ensures these devices are safe and work well.
Important Participants in the Downstream Distribution Chain
Downstream distributors are also important. They include:
- Medical equipment wholesalers
- Healthcare facility procurement networks
- Specialized cardiac care equipment retailers
- Global medical device distribution companies
Upstream suppliers and downstream distributors work together. This affects prices, availability, and new technologies in heart devices.
Strong partnerships and supply chain management are crucial. Manufacturers need to keep improving their chain to meet healthcare needs and tech expectations.
Innovation trends in miniaturization, biocompatibility, and AI-assisted devices
The world of mechanical circulatory support devices is changing fast. New technologies are focusing on three main areas: making devices smaller, more body-friendly, and using AI to manage them.
Miniaturization: Making Devices Smaller
Miniaturization is a big deal in device making. Scientists are working on smaller, less painful devices. These tiny technologies help doctors work more precisely and let patients recover faster.
- Reduced device size by up to 50% in recent prototypes
- Enhanced patient mobility and comfort
- Minimized surgical intervention requirements
Biocompatibility: Making Devices Better for the Body
Biocompatibility is another key area. New materials and treatments are making devices better for the body. The aim is to cut down on inflammation, prevent rejection, and make devices last longer.
| Innovation Area | Key Improvements | Patient Benefits |
| Material Engineering | Nano-structured surfaces | Reduced immune response |
| Surface Coatings | Anti-thrombogenic treatments | Lower risk of blood clots |
AI-Assisted Devices: Using AI to Manage Devices
AI is changing how we watch over patients and make devices work better. AI algorithms help with quick checks, predicting when devices might need fixing, and tailoring treatments. These smart tools give doctors a deeper look into how devices support the heart.
- Predictive maintenance algorithms
- Real-time performance monitoring
- Personalized treatment recommendations
The mix of miniaturization, biocompatibility, and AI is leading to smarter, more caring devices. These new devices are more advanced and better for patients than ever before.
Market restrictions from high cost, clinical complexity, and regulatory barriers
The Mechanical Circulatory Support Devices market faces big challenges. These come from high costs, clinical complexity, and strict rules. These issues affect healthcare providers, makers, and patients.
High costs are a big problem
Making and using these devices need a lot of money. Prices can go from $100,000 to $300,000. This is hard for hospitals and patients to handle.
- Research and development expenses exceed $50 million per device
- Clinical trial costs can reach $10-15 million
- Ongoing maintenance and specialized training add significant expenses
Clinical complexity is another big challenge
These devices need a lot of training for doctors. Teams must get special certifications to use them safely.
| Market Restriction Category | Primary Challenge | Estimated Impact |
| Financial Barriers | High Device Cost | 60% Market Limitation |
| Technical Complexity | Advanced Training Requirements | 25% Market Constraint |
| Regulatory Environment | Strict Approval Processes | 15% Market Restriction |
Regulatory barriers also slow things down
The FDA’s strict rules make it hard to bring devices to market. Makers have to spend a lot of time and money to show their devices are safe and work well.
These challenges make it hard to bring new technologies to patients. To fix this, we need everyone to work together. This includes device makers, healthcare teams, and rule makers.

Geopolitical impacts on healthcare funding and device approvals
The world of mechanical circulatory support devices is closely tied to global politics. How we fund healthcare and approve devices is influenced by international relations, trade rules, and investments in medicine.
Important factors affecting the mechanical circulatory support devices market include:
- Regulatory harmonization across international borders
- Strategic research collaborations between nations
- Economic sanctions affecting medical technology transfer
- Bilateral healthcare funding agreements
Different countries have their own ways of approving devices. The U.S. uses the FDA’s strict rules, while Europe uses the CE marking system. These differences make it hard for manufacturers to get their products on the global market.
How much money is spent on healthcare shows the impact of politics. Rich countries often spend more on new heart technologies, leading to uneven opportunities. But, poorer countries are starting to invest more in healthcare as heart disease grows and their health systems improve.
Working together is key in the complex world of medical device development. Partnerships between research groups, governments, and companies help navigate these challenges.
Understanding Mechanical Circulatory Support Devices
The world of mechanical circulatory support devices has made big strides in heart care. There are three main types: ventricular assist devices, total artificial hearts, and ECMO systems.
1. Ventricular Assist Devices (VADs)
Ventricular assist devices (VADs) are key in helping hearts that don’t work well. They come in two main types:
- Left ventricular assist devices (LVADs)
- Right ventricular assist devices (RVADs)
2. Total Artificial Hearts
Total artificial hearts are a full solution for those with no heart function left. These advanced devices replace the entire heart function, helping patients until they can get a transplant or live with the device long-term.
3. ECMO Systems
ECMO systems are a temporary but crucial help for those with severe heart or lung problems. These machines take over heart and lung work, giving organs a chance to heal or get ready for more lasting treatments.
| Device Type | Primary Function | Patient Application |
| Ventricular Assist Devices | Partial Heart Support | Heart Failure Patients |
| Total Artificial Hearts | Complete Heart Replacement | End-Stage Heart Failure |
| ECMO Systems | Temporary Organ Support | Critical Cardiac/Respiratory Failure |
Each device has its own benefits for dealing with tough heart issues. New tech keeps making them better, improving how well they work and the results for patients.
Application segmentation across heart failure, cardiogenic shock, and transplantation
Mechanical circulatory support devices have changed how we treat heart failure. They offer crucial help for those with severe heart problems.
These devices are used in several ways:
- Heart Failure Management
- Bridge-to-transplantation support
- Destination therapy for chronic patients
- Long-term cardiac assistance
- Cardiogenic Shock Management
- Emergency cardiac intervention
- Rapid hemodynamic stabilization
- Acute life-saving support
- Heart Transplantation Support
- Pre-transplant patient stabilization
- Post-transplant recovery assistance
- Bridging critical cardiac transitions
Cardiogenic shock management is a key area where these devices help. They prevent heart failure right away. Doctors use them for quick help during serious heart issues.
Support for heart transplantation has also improved a lot. These devices help patients live longer while waiting for a new heart. They have made a big difference in heart care by providing reliable help in tough situations.
New studies are finding more ways to use these devices. They promise better care and more tailored treatment for heart patients.
Global Regional Performance of Mechanical Circulatory Support Devices Market
The global market for mechanical circulatory support devices is complex. It shows different growth rates in various regions. Each area has its own way of adopting medical technology and building healthcare systems.
North America: Strong Market Due to Investments in Heart Care
In North America, the market is strong thanks to big investments in heart care. The growth here is fueled by advanced healthcare and lots of research funding. Key factors include:
- High prevalence of cardiovascular diseases
- Advanced medical research capabilities
- Strong reimbursement policies
- Technological innovation ecosystem
Asia: Rapid Growth in China and South Korea
Asian markets, especially in China and South Korea, are growing fast. These areas are quickly improving their medical tech sectors. They have more healthcare spending and are learning about new heart treatments.
Europe: Steady Growth with Adoption of New Technologies
In Europe, the market for these devices is growing steadily. Countries like Germany and the UK are adopting new heart care technologies. They have policies that support these innovations.
Latin America and the Middle East: Promise in Healthcare Investment
Latin America and the Middle East are also showing promise. They are investing in healthcare and technology. These regions will likely play a big role in the future market.
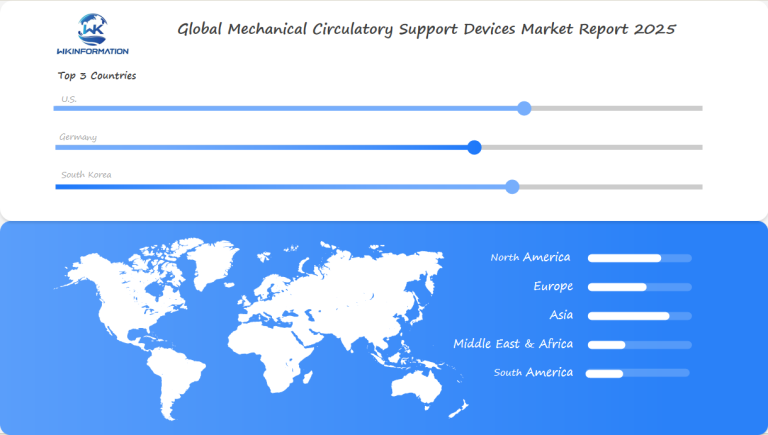
U.S. leadership in advanced cardiac therapies sustaining growth
The United States leads in the mechanical circulatory support devices market. This is thanks to its strong innovation ecosystem and advanced medical research. The country is at the forefront in creating life-saving cardiovascular technologies.
Several factors drive the U.S. market’s growth:
- Substantial investment in medical research and development
- Advanced healthcare infrastructure
- Supportive regulatory frameworks
- High healthcare expenditure
Medical institutions and research centers in the U.S. are key. They push the limits of mechanical circulatory support technologies. The U.S. Food and Drug Administration’s quick approval of new devices helps speed up the development of cardiac assist technologies.
The competitive landscape is shaped by partnerships between top medical device makers, universities, and healthcare providers. Continuous technological advancements in miniaturization and biocompatibility have strengthened the U.S. market’s global leadership in mechanical circulatory support devices.
Germany's reimbursement policies encouraging device adoption
The Germany market leads in mechanical circulatory support device innovation. This is thanks to forward-thinking healthcare policies that boost adoption rates. Germany’s detailed reimbursement strategies have built a strong base for advanced cardiac technologies.
Germany’s approach to mechanical circulatory support devices includes:
- Comprehensive public healthcare coverage for innovative cardiac technologies
- Streamlined approval processes for cutting-edge medical devices
- Substantial government funding for cardiovascular research
Germany healthcare institutions have a well-thought-out system. They balance cost-effectiveness with patient access. The national reimbursement policies prioritize patient outcomes and technological advancement. This creates a supportive environment for medical device makers and healthcare providers.
Research centers and hospitals in big Germany cities like Munich, Berlin, and Frankfurt are key. They work together to drive device development. Their teamwork between doctors, researchers, and tech developers leads to ongoing innovation and better patient care.
The Germany market’s strategy is a model for other European countries. It influences regional device adoption strategies and sets new standards in cardiovascular medical technology.
South Korea's technology-driven healthcare expansion fueling demand
The South Korean market is changing fast with new tech in mechanical circulatory support devices. As a top leader in medical innovation, South Korea is leading the way in technology-driven healthcare. This is changing how we care for hearts.
What’s driving the demand in South Korea?
Several factors are contributing to the increasing demand for mechanical circulatory support devices in South Korea:
- A rapidly aging population leading to more heart disease
- Big investments from the government in medical research
- Strong teamwork between medical places and global device makers
- Advanced tech supporting medical breakthroughs
South Korean research centers and medical schools are working on new mechanical circulatory support tech. Their focus on precision engineering and biomedical research has built a strong base for advanced heart care.
Partnerships between South Korean device companies and international research groups are speeding up innovation. These teams are creating smaller, more effective devices. These devices aim to improve patient results.
South Korea’s focus on tech in healthcare is opening up big market chances. This makes South Korea a major player in the global market for mechanical circulatory support devices.
The Future of Mechanical Circulatory Support Devices
The world of mechanical circulatory support devices is changing fast. New devices are making big leaps in medical tech. They bring real-time insights into how well the device and patient are doing.
How Remote Monitoring is Changing Cardiac Care
Remote monitoring is changing how doctors keep an eye on cardiac support systems. These new tools offer big benefits:
- Continuous patient health tracking
- Immediate detection of potential device complications
- Reduced hospital readmission rates
- Personalized patient care strategies
The Role of Medical Engineers in Developing Next-Generation Devices
Medical engineers are working on next-generation devices. These devices will have many diagnostic functions. They use artificial intelligence and machine learning to spot health risks early and improve device performance.
| Technology Feature | Patient Benefit |
| AI-Enhanced Monitoring | Predictive Health Insights |
| Real-Time Data Transmission | Immediate Clinical Intervention |
| Wireless Diagnostic Capabilities | Reduced Physical Monitoring |
The mix of integrated diagnostics and remote monitoring is a big step forward. Patients get more tailored, proactive care. This care is less invasive and more effective.
These new technologies will cut healthcare costs and improve patient results. They will also lead to quicker, more effective medical actions.
Key players and competitive strategies in the cardiac assist device space
Key Players:
-
Abbott Laboratories – USA
-
Medtronic – Ireland
-
Getinge (Maquet) – Sweden
-
LivaNova – United Kingdom
-
SynCardia Systems – USA
-
Abiomed – USA
-
Berlin Heart – Germany
-
Carmat – France
-
VAD (Ventricular Assist Device) companies – Various
-
Jarvik Heart – USA
Strategic approaches define the competitive landscape. These include:
- Aggressive research and development investments
- Strategic industry partnerships
- Global market expansion initiatives
- Advanced technological integration
New startups are shaking things up by bringing in new solutions. They focus on:
- Developing miniaturized device technologies
- Reducing overall treatment costs
- Enhancing patient outcomes
- Creating AI-integrated monitoring systems
Important partnerships are changing the mechanical circulatory support devices world. These partnerships between device makers, research groups, and healthcare providers speed up innovation. They also make devices work better.
The competition keeps getting fiercer. Market leaders are pouring money into the next big things. They aim to improve patient care and offer more treatment options.
Overall
| Report Metric | Details |
|---|---|
| Report Name | Global Mechanical Circulatory Support Devices Market Report |
| Base Year | 2024 |
| Segment by Type |
· Ventricular Assist Devices · Total Artificial Hearts · ECMO Systems |
| Segment by Application |
· Hospitals · Ambulatory Surgical Centers · Specialty Cardiology Centers · Others |
| Geographies Covered |
· North America (United States, Canada) · Europe (Germany, France, UK, Italy, Russia) · Asia-Pacific (China, Japan, South Korea, Taiwan) · Southeast Asia (India) · Latin America (Mexico, Brazil) |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
The Mechanical Circulatory Support Devices market is set to grow a lot. By 2025, it’s expected to see big increases. New technologies and more heart disease cases are pushing the market forward.
Experts say the market will hit about $3.45 billion. This shows big steps forward in heart care technology.
Looking ahead, we’ll see smaller and more tailored medical devices. Asia and other growing areas will add a lot to the market. Companies like Medtronic and Abbott are working hard to make these devices better.
Rules and how insurance pays for these devices will shape the market. Health systems around the world are seeing the value of these devices. New engineering, AI, and better materials will make heart treatments safer and more available.
The future looks bright for this market. Heart diseases are a big challenge, but these devices offer a solution. More research, partnerships, and acceptance by doctors will keep the market growing.
Global Mechanical Circulatory Support Devices Market Report (Can Read by Free sample) – Table of Contents
Chapter 1: Mechanical Circulatory Support Devices Market Analysis Overview
- Competitive Forces Analysis (Porter’s Five Forces)
- Strategic Growth Assessment (Ansoff Matrix)
- Industry Value Chain Insights
- Regional Trends and Key Market Drivers
- Mechanical Circulatory Support Devices Market Segmentation Overview
Chapter 2: Competitive Landscape
- Global Mechanical Circulatory Support Devices Players and Regional Insights
- Key Players and Market Share Analysis
- Sales Trends of Leading Companies
- Year-on-Year Performance Insights
- Competitive Strategies and Market Positioning
- Key Differentiators and Strategic Moves
Chapter 3: Mechanical Circulatory Support Devices Market Segmentation Analysis
- Key Data and Visual Insights
- Trends, Growth Rates, and Drivers
- Segment Dynamics and Insights
- Detailed Market Analysis by Segment
Chapter 4: Regional Market Performance
- Consumer Trends by Region
- Historical Data and Growth Forecasts
- Regional Growth Factors
- Economic, Demographic, and Technological Impacts
- Challenges and Opportunities in Key Regions
- Regional Trends and Market Shifts
- Key Cities and High-Demand Areas
Chapter 5: Mechanical Circulatory Support Devices Emerging and Untapped Markets
- Growth Potential in Secondary Regions
- Trends, Challenges, and Opportunities
Chapter 6: Product and Application Segmentation
- Product Types and Innovation Trends
- Application-Based Market Insights
Chapter 7: Mechanical Circulatory Support Devices Consumer Insights
- Demographics and Buying Behaviors
- TargetAudience Profiles
Chapter 8: Key Findings and Recommendations
- Summary of Mechanical Circulatory Support DevicesMarket Insights
- Actionable Recommendations for Stakeholders
Access the study in MULTIPLEFORMATS
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1-866-739-3133
Email: infor@wkinformation.com
What are Mechanical Circulatory Support Devices?
Mechanical Circulatory Support Devices are advanced medical devices used to assist patients with severe heart failure. They work by helping the heart pump blood effectively. These devices include:
- Ventricular Assist Devices (VADs)
- Total Artificial Hearts
- Extracorporeal Membrane Oxygenation (ECMO) systems
These devices play a crucial role in maintaining blood circulation and providing support to the heart.
How big is the Mechanical Circulatory Support Devices market expected to grow?
The market is expected to grow to $3.45 billion by 2025. This growth is due to more heart diseases, new technologies, and a need for less invasive treatments.
What are the primary clinical applications of these devices?
These devices are mainly used for advanced heart failure. They help in the following ways:
- As a bridge to transplant
- For destination therapy
- In treating shock
They also support patients during and after heart transplants.
Which countries are leading in Mechanical Circulatory Support Device development?
The United States is leading with its strong research and healthcare tech. Germany is key in Europe thanks to good insurance policies. South Korea is also growing fast with new cardiac tech.
What are the main challenges in the Mechanical Circulatory Support Devices market?
The Mechanical Circulatory Support Devices market faces several significant challenges:
- High Costs: The cost of manufacturing and implementing these devices can be quite high, which may limit their accessibility and adoption in certain healthcare settings.
- Complex Use: The operation and management of these devices can be complex, requiring specialized training for healthcare professionals.
- Strict Regulations: There are stringent regulations governing the approval and use of medical devices in various countries. This can create barriers to entry for new players in the market.
- Diverse Healthcare Standards: Different countries have different healthcare standards and practices. This diversity can make it challenging for manufacturers to design products that meet the needs of multiple markets.
- Slow Growth: The combination of high costs, complex use, strict regulations, and diverse healthcare standards can result in slow growth for the Mechanical Circulatory Support Devices market.
These challenges need to be addressed in order to foster growth and innovation in this field.
How are artificial intelligence and technology improving these devices?
AI is changing the game by making device management better. It allows for real-time monitoring and remote checks. New tech aims to make devices smaller, more friendly to the body, and smarter.
What types of Mechanical Circulatory Support Devices exist?
There are left and right VADs, Total Artificial Hearts, and ECMO systems. Each type meets different needs and conditions for patients.
What future trends are expected in this market?
Future trends include better diagnostics, remote monitoring, and personalized care. Devices will get smaller and safer. There will be a focus on fewer complications and better patient results.

