Central Lab Services Market Anticipated to Hit $5.97 Billion by 2025: Clinical Trial Demand Surges in the U.S., Germany, and Japan
Explore the evolving landscape of the Global Central Lab Services Market from 2025-2033. This comprehensive analysis examines key growth drivers, technological innovations, and regional dynamics shaping the industry. Learn how automation, AI/ML integration, and increasing clinical trials are transforming laboratory operations worldwide. Discover market trends, competitive strategies, and future prospects in this essential sector of healthcare and pharmaceutical development.
- Last Updated:
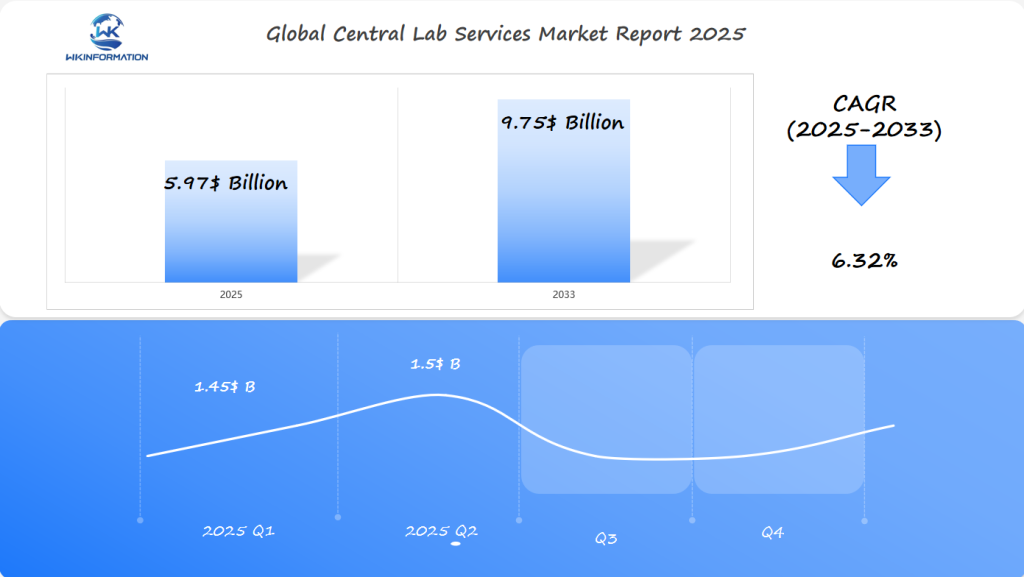
Central Lab Services Market Forecast for Q1 and Q2 2025
The central lab services market is set to reach $5.97 billion by 2025, expanding at a CAGR of 6.32% from 2025 to 2033. The U.S. continues to lead in the demand for centralized laboratory services, driven by advancements in medical research, diagnostics, and the growing need for streamlined clinical trials. Germany’s strong healthcare infrastructure, along with Japan’s aging population, is contributing to the increasing demand for diagnostic testing and laboratory support services.
Q1 2025 sales are expected to be approximately $1.45 billion, with slight growth to $1.5 billion in Q2, as the adoption of high-throughput technologies and outsourcing of lab operations continue to grow in the healthcare and pharmaceutical sectors.
Upstream and Downstream Structure in Biopharma R&D Services
The world of biopharma R&D is complex. Upstream and downstream services are key to moving medical research forward. These steps are essential for creating new drugs and advancing clinical trials.
Upstream Services
Upstream services start early in research and development. They include important steps like:
- Target identification and validation
- Molecular screening
- Preclinical research
- Initial compound development
Downstream Services
Downstream services focus on later stages. They cover:
- Clinical trial management
- Data analysis and interpretation
- Regulatory submission preparation
- Final drug formulation
The connection between upstream and downstream services is vital. It helps speed up medical discoveries. Central lab services are key in linking these steps. They ensure smooth communication and accurate data throughout the research process.
Combining upstream and downstream services well can cut down development time. It also boosts research efficiency.
Pharmaceutical companies now see the value in improving these services. They aim to innovate faster and bring new treatments to market sooner.
Trends in Outsourcing and Centralization of Lab Testing
The pharmaceutical and biotechnology industries are changing fast. They are moving towards more centralized lab testing. This change makes clinical trials smoother and more efficient.
What’s driving this change?
Several factors are at play:
- Cost reduction through economies of scale
- Standardization of testing methodologies
- Enhanced data quality and integrity
- Access to specialized expertise
Centralization is changing how companies handle lab services. By bringing testing under one roof, they get better consistency. This also helps reduce the mess in research processes.
| Outsourcing Trend | Impact on Lab Testing | Potential Benefits |
| Centralized Lab Services | Standardized Protocols | Improved Data Reliability |
| Strategic Partnerships | Advanced Technical Capabilities | Cost-Effective Solutions |
| Global Network Integration | Expanded Research Reach | Accelerated Clinical Trials |
New lab testing models are emerging. They let research groups focus on their main work. At the same time, they use experts for specific tasks. This makes research faster and more flexible.
Regulatory Hurdles and Data Integrity Challenges
Clinical trials face many regulatory challenges that need careful attention to data integrity. Central lab services are key in dealing with these complex rules of clinical research.
To meet regulatory standards, several strategies are important:
- Implementing rigorous quality control protocols
- Ensuring consistent documentation practices
- Developing standardized data collection methods
- Conducting regular compliance audits
Pharmaceutical companies find it tough to manage multi-site research projects. Data integrity is crucial for reliable research outcomes. Bodies like the FDA and EMA set strict rules to ensure research quality and patient safety.
| Regulatory Challenge | Mitigation Strategy | Impact on Trials |
| Documentation Consistency | Centralized Data Management | Improved Research Accuracy |
| Sample Tracking | Advanced Tracking Systems | Enhanced Chain of Custody |
| Compliance Verification | Regular External Audits | Reduced Regulatory Risk |
Central labs use advanced technologies to meet these regulatory needs. By standardizing procedures and using strong data management systems, researchers can lower risks in clinical trials.

Geopolitical Factors Shaping Global Clinical Trial Distribution
The world of global clinical trials is getting more complex. This is because of many geopolitical factors that affect where trials are done. Researchers and drug companies face a tough international setting when picking places for their studies.
Important Geopolitical Factors in Global Clinical Trials
Some of the key geopolitical factors influencing global clinical trials include:
- Regulatory compliance across different national frameworks
- Political stability of potential research regions
- Healthcare infrastructure capabilities
- Economic investment in medical research
Emerging Markets as Attractive Locations for Clinical Trials
Emerging markets are now very appealing for clinical trials. Countries like India, China, and Brazil stand out. They have big patient groups, lower costs, and better medical systems.
| Region | Clinical Trial Attractiveness | Key Challenges |
| North America | High regulatory standards | Expensive research costs |
| Asia-Pacific | Large patient pools | Variable regulatory environments |
| Eastern Europe | Cost-effective recruitment | Political uncertainty |
Choosing the right places for trials needs careful thought. Pharmaceutical companies must balance scientific needs with complex international factors. They aim to make global trials work well while keeping research standards high and avoiding problems.
Knowing these geopolitical details helps in making better, worldwide clinical trials. This speeds up medical progress and helps patients more.
Segmentation by Service Type: Biomarker Testing, Logistics, and Project Management
The central lab services market is growing fast. It’s thanks to key service segments that push clinical research forward. Biomarker testing is a big deal, helping researchers create better treatments and tests for many diseases.
Important service types in the central lab world include:
- Biomarker testing with advanced molecular analysis techniques
- Clinical trial logistics management
- Comprehensive project management solutions
Biomarker testing is a smart way to understand diseases. Researchers use these tests to find special markers in the body. Precision medicine needs accurate biomarker testing to work well.
Clinical trial logistics are key to making research run smoothly. They handle everything from collecting samples to keeping them safe. Project management keeps all the moving parts working together, making sure everyone is on the same page.
These services together change clinical research for the better. They:
- Make diagnosing diseases more accurate
- Make research easier to manage
- Help get new treatments to patients faster
New tech is changing how we do biomarker testing, manage trials, and plan projects. More and more, research groups and drug makers see the value in these services. They help drive new discoveries in medicine.
Applications in Oncology, Rare Diseases, and Vaccine Development
Central lab services play a key role in medical research. They are crucial in oncology trials, helping find new treatments and diagnostics. Researchers use advanced molecular tests to create targeted cancer therapies.
Oncology Trials
Central lab services are essential in oncology trials for:
- Finding new cancer treatments
- Developing better cancer diagnostic methods
Rare Disease Research
Rare disease research is also growing fast. Companies are spending a lot to understand these diseases better. This includes conditions like Huntington’s disease and cystic fibrosis. Central lab services are essential here, offering:
- Advanced genetic sequencing technologies
- Comprehensive biomarker identification
- Precision diagnostic screening
Vaccine Development
Vaccine development is another area where central lab services are vital. The COVID-19 pandemic showed how important fast, reliable vaccine research is. Now, oncology trials and vaccine development use advanced lab networks for:
- Streamlined clinical trial protocols
- Enhanced data management systems
- Rigorous quality control processes
The integration of cutting-edge technologies and specialized expertise positions central lab services as fundamental drivers of medical innovation across diverse research landscapes.
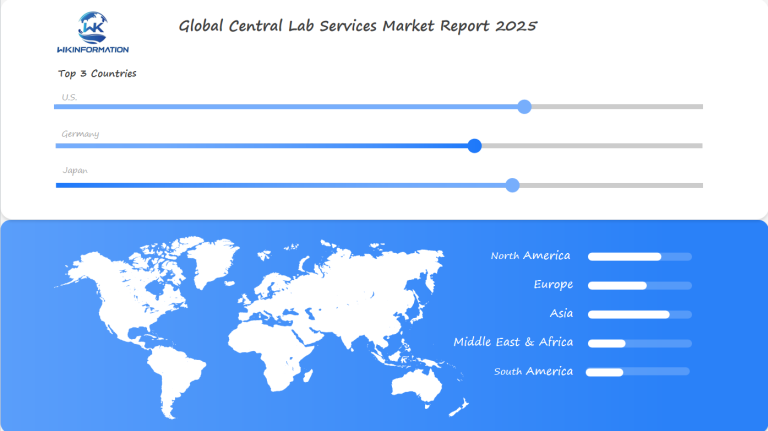
Understanding the Demand for Central Lab Services in Different Regions
The world of central lab services is complex. It’s shaped by where people live and the needs of clinical research. Market geography is key in guiding how drugs are developed across the globe.
Several factors affect where central lab services are needed:
- Regulatory environments
- Research infrastructure
- Pharmaceutical investment levels
- Healthcare technology advancement
Pharmaceutical companies are now more careful about where they do clinical trials. The United States, Europe, and Asia-Pacific are the main places for central lab service demand. Each area has its own strengths, making the global market dynamic and connected.
“Regional capabilities determine the efficiency and effectiveness of clinical trial processes” – Pharmaceutical Research Consortium
New markets are growing fast in central lab services. Countries like China, India, and Brazil are spending a lot on research. They’re becoming strong contenders for global clinical trials.
The need for central lab services keeps changing. It’s driven by new tech, more drugs in development, and complex research.
U.S. Drives Growth with Expanding Drug Pipelines
The U.S. leads the world in clinical trial services. This is thanks to a strong mix of research, innovation, and investment in drug development.
Several factors drive the U.S. drug pipelines forward:
- Substantial research funding from government and private sectors
- Advanced technological infrastructure
- Sophisticated regulatory frameworks
- Strong intellectual property protections
Pharmaceutical companies are now focusing on personalized medicine and complex disease research. The U.S. is well-equipped to handle complex clinical trials in many areas.
| Market Segment | Growth Percentage | Key Characteristics |
| Oncology Trials | 18.5% | Precision targeting, molecular diagnostics |
| Rare Disease Research | 12.3% | Advanced genomic screening |
| Infectious Disease Trials | 15.7% | Rapid response capabilities |
The U.S. drug pipelines draw in global investments, promising continued growth and innovation in pharmaceutical research.
Germany's Regulatory Strength and Pharma Innovation
The German pharmaceutical industry is a top player in European medical research. It’s a key spot for central lab services. Companies and researchers use Germany’s strong rules to push the limits of clinical trials and science in the complex European regulatory landscape.
Germany has several strengths in pharmaceutical development:
- Exceptional regulatory compliance standards
- High-quality research infrastructure
- Significant investment in biotechnology
- Advanced technological capabilities
Germany is a top choice for clinical research thanks to its strict rules. Pharmaceutical companies see its advanced healthcare and research networks as big pluses for complex medical studies.
What drives Germany’s pharmaceutical innovation includes:
- Substantial government funding for research
- Strong collaboration between academic institutions and industry
- Stringent quality control mechanisms
- Sophisticated data management protocols
International pharmaceutical organizations see Germany as a top spot for new treatments. This is especially true for complex areas like oncology and rare diseases. Germany’s innovation hubs draw global research investments, making it a leading center for pharmaceutical progress.
Japan's Aging Population Encourages Trial Activity
Japan leads in clinical research thanks to its unique population. The growing number of older people is driving more trials in healthcare. Researchers are working hard to find new ways to diagnose and treat age-related diseases.
The growth of clinical research in Asia is boosted by Japan. Pharmaceutical companies see Japan’s strong healthcare and advanced technology as big pluses. This makes it a great place for complex clinical trials.
- Rapidly aging population creates unique research opportunities
- High medical technology standards support advanced clinical research
- Increased funding for geriatric medical studies
Japan’s aging population brings special challenges and chances for research. The high number of elderly people offers a chance to develop targeted treatments. Researchers are looking into:
- Neurological disorder treatments
- Cardiovascular disease interventions
- Age-related chronic condition management
Central lab services are growing fast as companies use Japan’s vast research network. The aging population is key to improving clinical trials and finding new medical solutions.
Japan’s demographic challenges are transforming into research opportunities in the global clinical trials landscape.
Future Outlook for AI Integration and Remote Trials
The world of clinical trials is changing fast. New remote trial tech and AI are leading the way. They help make research easier, cheaper, and more accurate.
Big changes are coming to clinical research:
- Advanced AI algorithms for predictive patient recruitment
- Real-time data monitoring through decentralized trial platforms
- Precision medicine approaches using machine learning
- Enhanced patient engagement through digital health technologies
“AI and remote technologies are not just improving clinical trials—they’re reimagining the entire research paradigm.” – Clinical Research Innovation Report, 2023
Remote trials are making research more accessible. Especially for those in remote or underserved areas. Artificial intelligence algorithms can now predict potential trial participants, optimize study designs, and dramatically reduce screening timelines.
Competitive Landscape in Central Lab Contract Services
The central lab services market is very competitive. Many big players are working together to grow their share. They use new ideas and special services to stand out.
Key players in the central lab services world include:
- Laboratory Corporation of America Holdings (Labcorp) – United States
- Quest Diagnostics – United States
- Charles River Laboratories International, Inc. – United States
- ICON plc – Ireland
- Eurofins Scientific – Luxembourg
- Thermo Fisher Scientific Inc. – United States
- Medpace – United States
- Q² Solutions – United States
- Syneos Health – United States
- SGS – Switzerland
Overall
| Report Metric | Details |
|---|---|
| Report Name | Global Central Lab Services Market Report |
| Base Year | 2024 |
| Segment by Type |
|
| Segment by Application |
|
| Geographies Covered |
|
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
The central lab services market is set for big growth. Experts say it will grow at a 6.32% CAGR. This will take it from US$5.97 billion in 2025 to US$9.75 billion by 2033. This growth comes from more pharmaceutical research and new tech in healthcare worldwide.
Investing in this area is very appealing. New areas like biomarker testing and advanced clinical trial logistics are full of potential. The market research shows big growth in places with strong pharmaceutical industries, like the US, Germany, and Japan.
New tech and more complex research open up new ways for growth. Investors can look into special central lab services that use AI, remote trials, and better data management. The market’s chance for growth and innovation makes it a great place for smart investments.
But, investors need to think carefully. The market’s growth looks good, but there are risks like tough regulations and needing to keep up with tech. To succeed, investors should look for service providers that are flexible, tech-savvy, and know the global clinical research scene well.
Global Central Lab Services Market Report (Can Read by Free sample) – Table of Contents
Chapter 1: Central Lab Services Market Analysis Overview
- Competitive Forces Analysis (Porter’s Five Forces)
- Strategic Growth Assessment (Ansoff Matrix)
- Industry Value Chain Insights
- Regional Trends and Key Market Drivers
- Central Lab ServicesMarket Segmentation Overview
Chapter 2: Competitive Landscape
- Global Central Lab Services players and Regional Insights
- Key Players and Market Share Analysis
- Sales Trends of Leading Companies
- Year-on-Year Performance Insights
- Competitive Strategies and Market Positioning
- Key Differentiators and Strategic Moves
Chapter 3: Central Lab Services Market Segmentation Analysis
- Key Data and Visual Insights
- Trends, Growth Rates, and Drivers
- Segment Dynamics and Insights
- Detailed Market Analysis by Segment
Chapter 4: Regional Market Performance
- Consumer Trends by Region
- Historical Data and Growth Forecasts
- Regional Growth Factors
- Economic, Demographic, and Technological Impacts
- Challenges and Opportunities in Key Regions
- Regional Trends and Market Shifts
- Key Cities and High-Demand Areas
Chapter 5: Central Lab Services Emerging and Untapped Markets
- Growth Potential in Secondary Regions
- Trends, Challenges, and Opportunities
Chapter 6: Product and Application Segmentation
- Product Types and Innovation Trends
- Application-Based Market Insights
Chapter 7: Central Lab Services Consumer Insights
- Demographics and Buying Behaviors
- Target Audience Profiles
Chapter 8: Key Findings and Recommendations
- Summary of Central Lab Services Market Insights
- Actionable Recommendations for Stakeholders
Access the study in MULTIPLEFORMATS
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1-866-739-3133
Email: infor@wkinformation.com
What is driving the growth of the Central Lab Services Market?
The market is growing fast due to several factors:
- Increase in rare diseases
- Government investments in research and development (R&D)
- Advancements in technology
- Rise in clinical trials conducted in countries like the U.S., Germany, and Japan
How do upstream and downstream processes impact central lab services?
Upstream and downstream processes are key in biopharma R&D. They make clinical trials more efficient and effective. They cover everything from sample collection to data analysis.
Why are pharmaceutical companies outsourcing lab testing?
Companies outsource lab testing for many reasons. They want standard processes, cost savings, better data, and to focus on research. Central labs provide consistent and reliable testing.
What regulatory challenges do central labs address?
Central labs tackle complex regulations. They ensure data integrity and maintain testing standards. They also help with compliance in various global markets.
How do geopolitical factors influence central lab services?
Geopolitical factors such as regulations, healthcare policies, demographics, and local pharmaceutical industry have an impact on central lab services. These factors determine the locations and extent of demand for these services.
What are the key service segments in central lab services?
Key services include:
- Biomarker testing
- Logistics
- Project coordination
- Sample processing
- Data analysis
- Specialized testing
These services are particularly important for areas such as oncology and rare diseases.
Which therapeutic areas are driving demand for central lab services?
Areas like oncology, rare diseases, vaccine development, personalized medicine, and complex chronic conditions are driving demand. Precision medicine is also a big factor.
Why is the U.S. market important for central lab services?
The U.S. is a leader because of its strong pharmaceutical industry, drug pipelines, healthcare system, regulations, and investment in research.
How are technological innovations impacting central lab services?
New technologies such as AI, machine learning, remote trials, and data analytics are transforming central lab services. These advancements are making research more efficient and accurate.
What are the future growth prospects for central lab services?
The market is expected to grow to $5.97 billion by 2025. This is due to more complex trials, R&D investment, tech advancements, and global healthcare needs.

