Immuno-Oncology Market Set to Reach $325.6 Million by 2025: Rapid Expansion in the U.S., Germany, and China
Explore comprehensive insights into the immuno-oncology market, projected to reach $325.6 million by 2025. This analysis covers key market trends, technological innovations, regional developments in the U.S., Germany, and China, and the competitive landscape shaping cancer immunotherapy’s future.
- Last Updated:
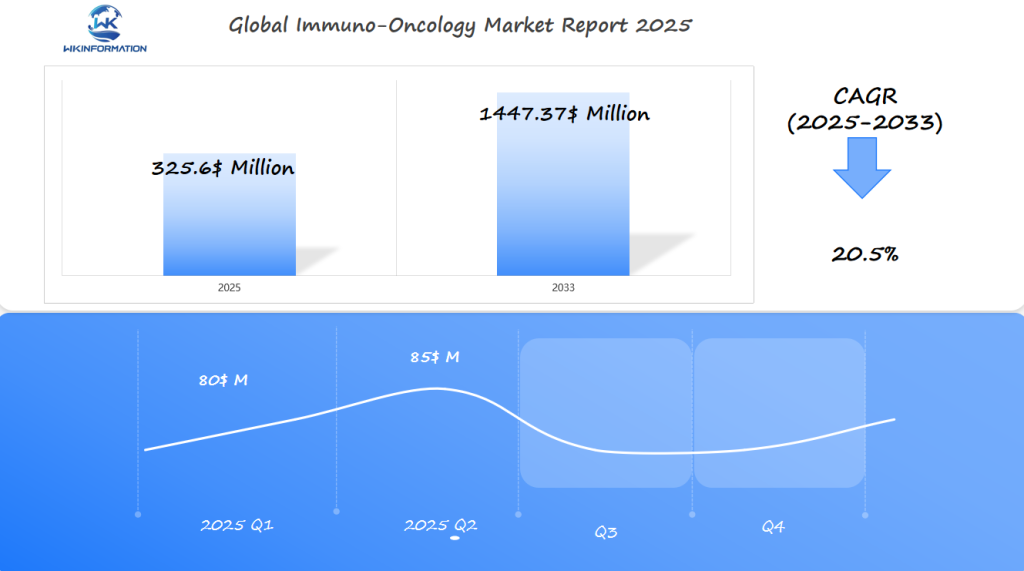
Immuno-Oncology Market Q1 and Q2 2025 Forecast
The Immuno-Oncology market is expected to reach $325.6 million in 2025, growing at a strong CAGR of 20.5% from 2025 to 2033. In Q1 2025, the market is projected to generate around $80 million, driven by increasing investment in cancer immunotherapy and the rapid growth of biopharma innovations in the field of immuno-oncology. The U.S., Germany, and China will lead the market, as they are home to major research institutions and pharmaceutical companies actively developing immunotherapy treatments.
By Q2 2025, the market will likely grow to around $85 million, as more immuno-oncology drugs and treatments receive approval from regulatory bodies, and biologic drugs continue to be launched for various types of cancer. Demand will also be fueled by increased clinical trials, breakthroughs in tumor-specific therapies, and growing partnerships between pharmaceutical companies and biotech startups focused on immune system therapies.
Understanding Immuno-Oncology Development: Upstream and Downstream Factors
Immuno-oncology therapies are developed through a combination of research and innovation. There are two main areas that influence this development: upstream factors and downstream factors.
Upstream Factors Driving Immuno-Oncology Development
Upstream factors refer to the elements that contribute to the creation and advancement of immuno-oncology therapies. These include:
- Advanced Research Technologies: Technologies that enhance the understanding of cancer biology and facilitate drug discovery.
- Next-generation sequencing
- Artificial intelligence for drug discovery
- Biomarker identification platforms
- Clinical Trial Infrastructure: The systems and resources in place to conduct clinical trials effectively.
- Specialized research facilities
- Patient recruitment networks
- Data management systems
Downstream Factors Shaping Patient Access to Treatments
Downstream factors, on the other hand, are the aspects that determine how these therapies are delivered to patients. They play a crucial role in ensuring that innovations reach those who need them most. Key downstream factors include:
- Treatment Delivery Systems: The mechanisms through which treatments are administered to patients.
- Specialized infusion centers
- Trained healthcare professionals
- Cold chain logistics
- Patient Access Mechanisms: The processes that govern patient access to treatments.
- Insurance coverage protocols
- Healthcare provider networks
- Treatment guidelines
Real-World Impact of Immuno-Oncology Therapies
Real-world data provides valuable insights into the effects of immuno-oncology therapies on patient outcomes. Here are some key findings:
- Improved Survival Rates: Targeted therapies have been associated with a significant increase in survival rates, with studies showing an improvement of up to 60%.
- Reduced Hospital Stays: Patients receiving targeted therapies often experience shorter hospital stays, with reductions of up to 40% reported.
- Enhanced Quality of Life Metrics: Various quality of life measures indicate positive outcomes for patients undergoing immuno-oncology treatments.
The Interplay Between Upstream Research and Downstream Delivery
The relationship between upstream research and downstream delivery is intricate. Pharmaceutical companies invest substantial resources into developing new therapies, while healthcare providers focus on implementing these treatments effectively.
This interplay creates a dynamic ecosystem where both drug development and patient care continuously evolve. It encourages collaboration between researchers, clinicians, and industry stakeholders to address challenges and improve outcomes.
Recent Breakthroughs in Immuno-Oncology Development
Recent advancements in upstream development have resulted in groundbreaking therapies such as CAR-T cells and bi-specific antibodies. These innovations directly impact downstream factors by necessitating the establishment of specialized treatment centers and training programs for healthcare providers.
By understanding the upstream and downstream influences on immuno-oncology development, we can better appreciate the complexities involved in bringing these therapies from the lab to the clinic.
Market Trends Shaping the Future of Immuno-Oncology
The field of immuno-oncology is undergoing significant changes with the introduction of new and innovative treatment methods. One such method, known as checkpoint inhibitors, has proven to be a game-changer in cancer therapy. These drugs work by targeting specific proteins, such as PD-1 and CTLA-4, to boost the immune system’s ability to identify and destroy cancer cells.
Key immunotherapy innovations revolutionizing cancer treatment:
- CAR T-cell therapy – Engineered T-cells specifically designed to target cancer cells, showing remarkable success rates in blood cancers
- Bispecific antibodies – Novel molecules capable of simultaneously binding to T-cells and cancer cells
- Neoantigen vaccines – Personalized treatments based on individual tumor mutations
Combination therapies represent a significant shift in treatment protocols. Clinical data demonstrates enhanced efficacy when combining:
- Checkpoint inhibitors with conventional chemotherapy
- Multiple checkpoint inhibitors targeting different pathways
- Immunotherapy with targeted molecular therapies
Research indicates that combination approaches can increase response rates by 20-30% compared to monotherapy treatments. These synergistic effects are particularly notable in melanoma and lung cancer patients.
The integration of artificial intelligence in immunotherapy development is accelerating biomarker discovery and patient response prediction. Machine learning algorithms analyze complex immune response patterns, enabling more precise treatment selection and timing.
Overcoming Barriers to Immuno-Oncology Adoption
The immuno-oncology market faces significant challenges that impact widespread adoption. Drug resistance remains a critical concern, with approximately 60% of patients showing limited response to existing immunotherapy treatments. This resistance can develop through multiple mechanisms:
- Tumor mutation burden changes
- Altered immune recognition
- Immunosuppressive microenvironment development
The high cost of immunotherapy presents another substantial barrier. A single treatment course can range from $100,000 to $400,000, creating significant financial burdens for patients and healthcare systems.
Key Solutions to Address Market Challenges:
- Enhanced Financial Support ProgramsPatient assistance initiatives
- Insurance coverage expansion
- Cost-sharing arrangements
- Improved Patient EducationDigital awareness campaigns
- Healthcare provider training
- Community outreach programs
- Research and Development SolutionsBiomarker identification
- Resistance mechanism studies
- Alternative drug delivery methods
Healthcare providers are implementing personalized medicine approaches to combat drug resistance. This strategy involves genetic testing and biomarker analysis to identify patients most likely to respond to specific treatments.
The development of biosimilars promises to reduce treatment costs by 20-30%, making immunotherapy more accessible. Organizations are establishing patient support networks to navigate insurance coverage and financial assistance programs.

Geopolitical Impacts on Immuno-Oncology Therapy Expansion
International regulations create a complex landscape for immuno-oncology therapy development. Different approval requirements across regions can significantly impact time-to-market and development costs:
Key Regulatory Frameworks:
- FDA (United States): Stringent clinical trial requirements with accelerated approval pathways
- EMA (European Union): Centralized authorization process with specific documentation needs
- NMPA (China): Recent reforms streamlining approval processes for innovative therapies
Government policies directly influence market access through:
- Pricing ControlsReference pricing systems
- Value-based pricing negotiations
- National reimbursement decisions
- Research FundingPublic-private partnerships
- Academic research grants
- Innovation incentives
Trade relationships between nations affect technology transfer and intellectual property rights in immuno-oncology development. Recent global events have highlighted vulnerabilities in supply chains, pushing countries to strengthen domestic manufacturing capabilities.
Policy Impact Examples:
- Brexit’s influence on UK-EU drug approval processes
- US-China trade tensions affecting technology sharing
- Japan’s fast-track approval system for breakthrough therapies
Regional harmonization efforts, such as the International Council for Harmonisation (ICH), aim to standardize requirements across borders. These initiatives reduce duplicate testing requirements and accelerate global access to innovative immuno-oncology treatments.
Detailed Exploration of Immuno-Oncology Market Segmentation
The immuno-oncology market divides into distinct product categories, each serving specific therapeutic approaches:
1. Checkpoint Inhibitors
- PD-1/PD-L1 inhibitors
- CTLA-4 inhibitors
- LAG-3 inhibitors
2. Cancer Vaccines
- Preventive vaccines
- Treatment vaccines
- Personalized neoantigen vaccines
3. Cell Therapies
- CAR T-cell therapy
- NK cell therapy
- TIL therapy
4. Cytokines
- Interleukins
- Interferons
- Growth factors
The checkpoint inhibitors segment dominates the market share at 63%, with products like Keytruda and Opdivo leading sales. Cancer vaccines represent a growing segment, particularly in personalized medicine applications.
Cell therapies show promising growth rates, with CAR T-cell treatments demonstrating remarkable success in blood cancers. The cytokines segment maintains steady growth, primarily in combination therapy approaches.
Market analysis reveals emerging subsegments:
- Bispecific antibodies: Targeting multiple pathways simultaneously
- Oncolytic viruses: Engineered to selectively destroy cancer cells
- Small molecule immunomodulators: Enhancing immune system response
These product segments reflect evolving treatment strategies and technological capabilities in cancer immunotherapy. Each category addresses specific patient needs and cancer types, contributing to the market’s dynamic growth trajectory.
How Applications are Driving Immuno-Oncology Growth
Cancer Immunotherapy
Immuno-oncology therapies, such as checkpoint inhibitors (e.g., PD-1/PD-L1 inhibitors, CTLA-4 inhibitors), enhance the immune system’s ability to target and destroy cancer cells, revolutionizing treatments for cancers like melanoma, lung cancer, and more.
Personalized Medicine
Tailored treatments based on the genetic makeup of both the patient and the tumor are transforming cancer care. By using biomarkers and genomics, immuno-oncology therapies can be personalized for higher efficacy and fewer side effects.
Combination Therapies
The combination of immuno-oncology treatments with other therapies, including chemotherapy, targeted therapy, and radiotherapy, improves efficacy and extends the range of treatable cancers, offering better outcomes for patients.
Cancer Vaccines
Therapeutic vaccines stimulate the immune system to recognize and fight specific cancer cells, offering a promising avenue for treating cancers like prostate cancer and breast cancer. These vaccines are being developed to complement existing cancer therapies.
Adoptive Cell Therapy (ACT)
ACT treatments, such as CAR-T cell therapy, modify a patient’s T-cells to enhance their cancer-fighting ability. CAR-T therapy has shown remarkable success in treating blood cancers like leukemia and lymphoma.
Monoclonal Antibodies
Engineered antibodies that target cancer cells are a critical application. These antibodies can either directly kill cancer cells or mark them for destruction by the immune system, significantly improving treatment outcomes.
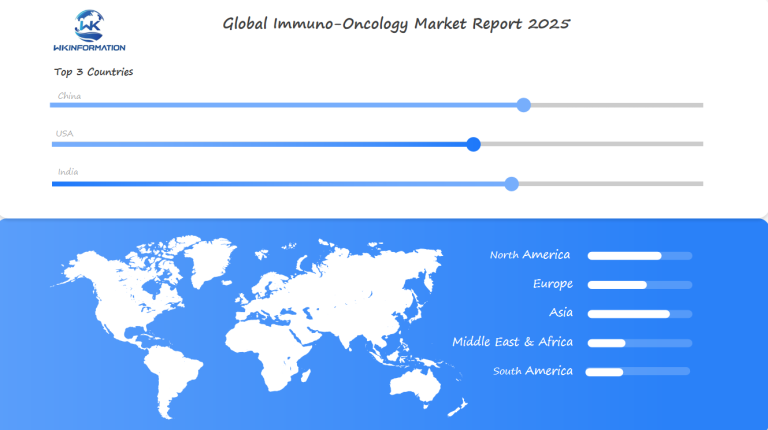
Global Regional Trends in the Immuno-Oncology Market
The immuno-oncology market shows clear regional trends, with North America holding a 45% market share in 2023. This dominance is due to:
- Advanced healthcare infrastructure
- High adoption rates of new therapies
- Strong presence of major pharmaceutical companies
- Supportive reimbursement policies
Europe is the second-largest market, with Germany leading regional growth at a CAGR of 24.3%. The European market benefits from:
- Extensive clinical research programs
- Strategic partnerships between biotech companies
- Government-backed innovation initiatives
Asia-Pacific is the fastest-growing region, driven by:
- Increasing healthcare spending
- Rising cancer rates
- Growing clinical trial activities
- Expanding medical tourism
Factors Influencing Regional Market Performance
Several factors impact the performance of immuno-oncology markets in different regions:
- Healthcare Infrastructure
- Quality of medical facilities
- Availability of skilled healthcare professionals
- Research and development capabilities
- Economic Factors
- Healthcare spending
- Insurance coverage
- Patient affordability
- Regulatory Environment
- Approval timelines
- Clinical trial requirements
- Market access policies
The Latin American and Middle Eastern markets show promising growth potential, with Brazil and Saudi Arabia emerging as key regional hubs for immuno-oncology research and treatment centers.
U.S. Immuno-Oncology Market: Growth Drivers and Innovations
The U.S. leads the global immuno-oncology market through strategic advantages in research, development, and clinical implementation. The market’s robust growth stems from several key factors:
Research Infrastructure Excellence
- Advanced research facilities and laboratories
- Strong academic-industry partnerships
- Substantial private sector investment
- Extensive clinical trial networks
The U.S. dominance in immune checkpoint inhibitors reflects breakthrough innovations by pharmaceutical giants like Merck and Bristol Myers Squibb. These companies have established successful treatment protocols for various cancer types, including melanoma, lung cancer, and bladder cancer.
Market Growth Catalysts
- FDA’s expedited approval pathways
- Comprehensive insurance coverage
- High healthcare spending
- Early adoption of innovative therapies
The U.S. healthcare system’s emphasis on precision medicine has accelerated the development of biomarker-driven immunotherapies. This approach enables targeted patient selection and improved treatment outcomes.
Innovation Highlights
- Development of next-generation checkpoint inhibitors
- Integration of artificial intelligence in drug discovery
- Advanced manufacturing capabilities
- Personalized therapy approaches
Recent breakthroughs in combination therapies have shown promising results, with U.S. research centers pioneering new treatment protocols that combine checkpoint inhibitors with conventional cancer treatments.
Germany's Role in Immuno-Oncology Development
Germany is a leader in immuno-oncology research and development, supported by its strong healthcare system and scientific expertise. The country’s research institutions have made significant breakthroughs in cancer immunotherapy.
Key Research Institutions Leading Innovation:
- German Cancer Research Center (DKFZ) in Heidelberg
- Max Planck Institute for Immunobiology
- Berlin Institute of Health
- University Hospital Frankfurt
German scientists have played a crucial role in the development of adoptive T-cell therapy and CAR-T cell research. The collaborative approach between academic institutions and pharmaceutical companies in Germany has sped up the process of innovation in immuno-oncology.
Notable Achievements:
- Development of novel antibody-drug conjugates
- Advanced tumor microenvironment research
- Pioneering work in personalized cancer vaccines
- Innovative checkpoint inhibitor combinations
The German government’s significant investment in biotechnology research has created a favorable environment for the advancement of immuno-oncology. Research funding through programs like the German Cancer Aid program has allowed for continuous innovation in cancer treatment methods.
German pharmaceutical companies have formed strong partnerships with international research organizations, promoting knowledge sharing and speeding up drug development timelines. These collaborations have led to several groundbreaking therapies currently being tested in clinical trials.
The existence of specialized cancer centers throughout Germany has made it easier to recruit participants for clinical trials and implement new immunotherapy protocols. This infrastructure supports both research progress and patient access to state-of-the-art treatments.
China's Expanding Immuno-Oncology Market Landscape
China’s immuno-oncology market has great potential for growth, with forecasts suggesting a significant market share by 2025. The country’s pharmaceutical industry has undergone significant changes through:
- Faster approval processes for innovative therapies
- Improved clinical trial infrastructure
- Strategic partnerships with global biotech companies
Investments in Immuno-Oncology Research
Local Chinese pharmaceutical companies have made substantial investments in immuno-oncology research, particularly in:
- CAR-T cell therapy development
- PD-1/PD-L1 inhibitors
- Novel checkpoint inhibitor combinations
Regulatory Reforms and International Interest
The National Medical Products Administration (NMPA) has implemented reforms to streamline the drug approval process, reducing waiting times from 3 years to 12 months for innovative cancer therapies. This regulatory shift has attracted international pharmaceutical companies to establish research centers in major Chinese cities.
Key Market Drivers
Key factors driving the market include:
- Rising cancer incidence rates
- Increased healthcare spending
- Growing middle-class population with better access to advanced treatments
- Government initiatives supporting biotech innovation
Emergence of Chinese Biotech Companies
Chinese biotech companies like BeiGene and Innovent Biologics have emerged as significant players in the immuno-oncology space, developing competitive products at lower costs. These companies leverage China’s large patient population for clinical trials, accelerating drug development timelines.
Areas of Strength in the Market
The market shows particular strength in:
- Biosimilar development
- Cost-effective treatment options
- Integration of traditional Chinese medicine with modern immunotherapy approaches
The Future of Immuno-Oncology: A Glimpse into 2025
The field of immuno-oncology is expected to undergo significant changes by 2025, thanks to innovative technologies and groundbreaking research. Industry experts predict major advancements in several key areas:
Anticipated Technological Developments:
- AI-powered drug discovery platforms will accelerate the development of targeted immunotherapies
- Personalized neoantigen vaccines tailored to individual tumor mutations
- Advanced biomarker detection methods for improved patient selection
- Integration of real-world data analytics for treatment optimization
Emerging Treatment Approaches:
- Next-generation CAR-T cell therapies with enhanced safety profiles
- Bispecific antibodies targeting multiple tumor antigens
- Novel checkpoint inhibitor combinations
- Microbiome-based immunotherapy solutions
Research suggests a shift towards precision medicine, where treatment plans are increasingly customized based on genetic analysis and immune system evaluation. The use of artificial intelligence in clinical decision-making will facilitate quicker and more precise treatment choices.
Market Evolution Indicators:
- 60% increase in clinical trials for combination therapies
- 40% reduction in manufacturing costs for cell therapies
- 35% improvement in patient response rates
- Expanded treatment options for previously resistant cancers
These developments mark a new chapter in cancer treatment, where immunotherapy becomes more widely available, effective, and tailored to each patient’s requirements.
Competitive Dynamics in the Immuno-Oncology Industry
The immuno-oncology market features several dominant players shaping the industry’s competitive landscape. Bristol Myers Squibb leads with its flagship drug Opdivo, generating substantial revenue through strategic partnerships and continuous research initiatives.
-
Bristol-Myers Squibb – United States
-
Merck & Co. – United States
-
Roche – Switzerland
-
AstraZeneca – United Kingdom
-
Pfizer Inc. – United States
-
Eli Lilly and Company – United States
-
GSK plc – United Kingdom
-
BeiGene Ltd. – China
-
Sanofi – France
-
Novartis AG – Switzerland
Overall
| Report Metric | Details |
|---|---|
| Report Name | Global Immuno-Oncology Market Report |
| Base Year | 2024 |
| Segment by Type |
· Checkpoint Inhibitors · Cancer Vaccines · Cell Therapies · Cytokines |
| Segment by Application |
· Cancer Immunotherapy · Personalized Medicine · Combination Therapies · Cancer Vaccines · Adoptive Cell Therapy (ACT) · Monoclonal Antibodies |
| Geographies Covered |
· North America (United States, Canada) · Europe (Germany, France, UK, Italy, Russia) · Asia-Pacific (China, Japan, South Korea, Taiwan) · Southeast Asia (India) · Latin America (Mexico, Brazil) |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
The immuno-oncology market represents a dynamic and rapidly evolving sector that continues to transform cancer treatment paradigms. Through detailed market segmentation analysis, we can observe the diverse therapeutic approaches and technologies that comprise this field. From checkpoint inhibitors to cell therapies, each segment contributes uniquely to the overall market landscape. This segmentation not only reveals current market dynamics but also highlights potential growth areas and investment opportunities.
As the field advances, understanding these distinct market segments becomes crucial for stakeholders to make informed decisions and optimize their market strategies. The continuous evolution of these segments, driven by technological innovations and clinical breakthroughs, ensures the immuno-oncology market remains at the forefront of cancer treatment development.
Global Immuno-Oncology Market Report (Can Read by Free sample) – Table of Contents
Chapter 1: Immuno-Oncology Market Analysis Overview
- Competitive Forces Analysis (Porter’s Five Forces)
- Strategic Growth Assessment (Ansoff Matrix)
- Industry Value Chain Insights
- Regional Trends and Key Market Drivers
- Immuno-OncologyMarket Segmentation Overview
Chapter 2: Competitive Landscape
- Global Immuno-Oncology players and Regional Insights
-
- Key Players and Market Share Analysis
- Sales Trends of Leading Companies
- Year-on-Year Performance Insights
- Competitive Strategies and Market Positioning
-
- Key Differentiators and Strategic Moves
Chapter 3: Immuno-Oncology Market Segmentation Analysis
- Key Data and Visual Insights
-
- Trends, Growth Rates, and Drivers
- Segment Dynamics and Insights
-
- Detailed Market Analysis by Segment
Chapter 4: Regional Market Performance
- Consumer Trends by Region
-
- Historical Data and Growth Forecasts
- Regional Growth Factors
-
- Economic, Demographic, and Technological Impacts
- Challenges and Opportunities in Key Regions
- Regional Trends and Market Shifts
- Key Cities and High-Demand Areas
Chapter 5: Immuno-Oncology Emerging and Untapped Markets
- Growth Potential in Secondary Regions
-
- Trends, Challenges, and Opportunities
Chapter 6: Product and Application Segmentation
- Product Types and Innovation Trends
- Application-Based Market Insights
Chapter 7: Immuno-Oncology Consumer Insights
- Demographics and Buying Behaviors
-
- Target Audience Profiles
Chapter 8: Key Findings and Recommendations
- Summary ofImmuno-Oncology Market Insights
- Actionable Recommendations for Stakeholders
Access the study in MULTIPLEFORMATS
Didn’t find what you’re looking for?
TALK TO OUR ANALYST TEAM
Need something within your budget?
NO WORRIES! WE GOT YOU COVERED!
Call us on: +1-866-739-3133
Email: infor@wkinformation.com
What are the key trends shaping the future of immuno-oncology?
Current trends in cancer immunotherapy include innovations in checkpoint inhibitors and adoptive cell transfer, as well as the influence of combination therapies on treatment efficacy.
What barriers exist in the adoption of immuno-oncology therapies?
Challenges facing the immuno-oncology market include drug resistance issues and high costs. Strategies are being developed to improve patient access and increase awareness about these treatments.
How do geopolitical factors impact the development of immuno-oncology therapies?
International regulations significantly affect therapy development and approval processes, with government policies playing a crucial role in shaping market dynamics.
What is the current state of China’s immuno-oncology market?
China’s immuno-oncology market is expanding rapidly, with numerous opportunities for growth influenced by its regulatory environment and increasing research activities.
Who are the major players in the immuno-oncology industry?
Key players in the immuno-oncology industry include Bristol Myers Squibb and Merck & Co., who are leading advancements in immune checkpoint inhibitors and other therapies.

